Stripping and distillation
Stripping (also called distillation) allows the separation of a solvent. This technology is often used to complement an evaporation unit for separating a solvent of produced condensates.
The most common applications being the separation of ammonia or alcohol, to improve on the quality of condensates.
Optimize energy efficiency of stripping
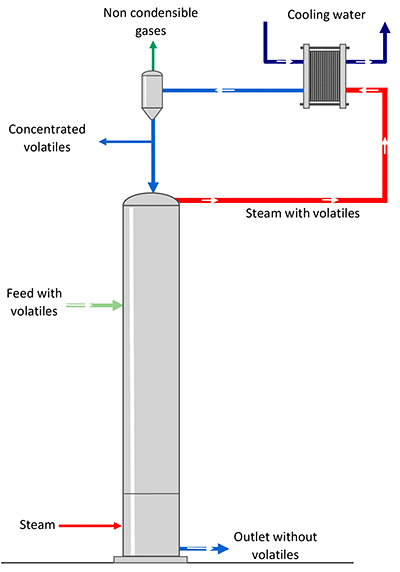
The stripping vapour needed to separate the volatile element of the product. Several technologies can reduce energy consumption related to this process.
Depending on your project, the desired capacity and the costs of available energy, France Evaporation will help you select the best technology, to optimize working costs and your investments.
Energy recovery at more than 100°C as hot water to supply stripping
In case an energy source higher than 100°C is available on site, it is reused and transferred in the form of hot water and then flashed to be transformed into vapour and to supply the stripping.
Mechanical Vapour Compression
France Evaporation patented technology, we developed a pattern consisting of a stripping, a MVC and a heat exchanger to reduce the consumption of vapour and optimize the working cost.
Thermo-compressor
Solvent outlet: case of ammonia
The solvent (ammonia for example) separated may be managed in several ways:
- production in concentrated form
- solvent destroyed by oxidation
- used to make denox
- production of salt (ammonium sulphate)
Production of ammonium sulphate and other saline solutions
It is possible to use a reactor in line with stripping to produce a saline solution in liquid form.
In the case of ammonia for example, France Evaporation proposes a production of salt in liquid form, directly at the stripping outlet.
For example, it is possible to produce ammonium sulphate at the stripping outlet to:
- use as fertilizer
- obtain a stable product that is easier to store
- access a resale market of the more open solution
- manage the thermal acid-based reaction directly in the unit






